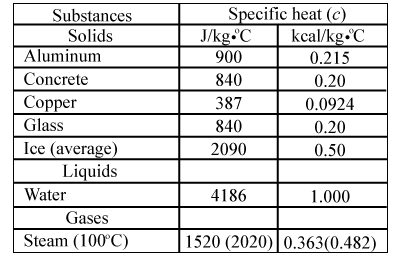
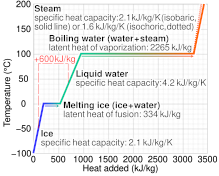
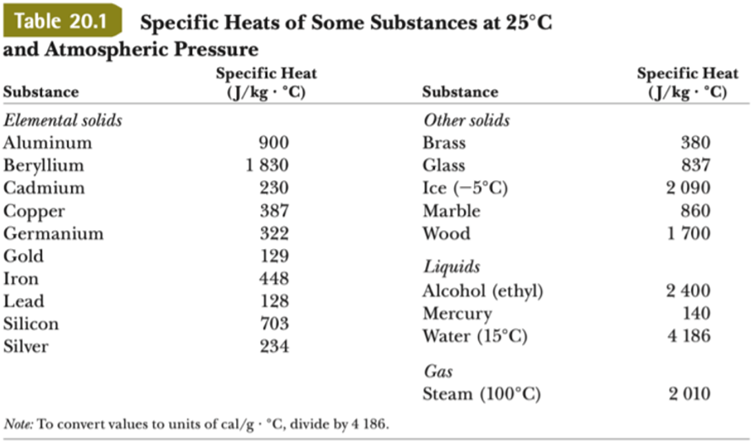
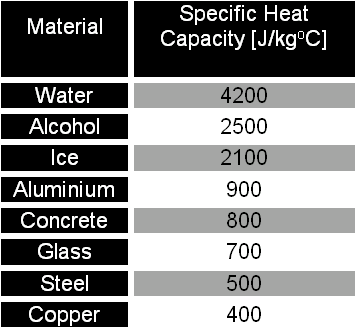
Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×
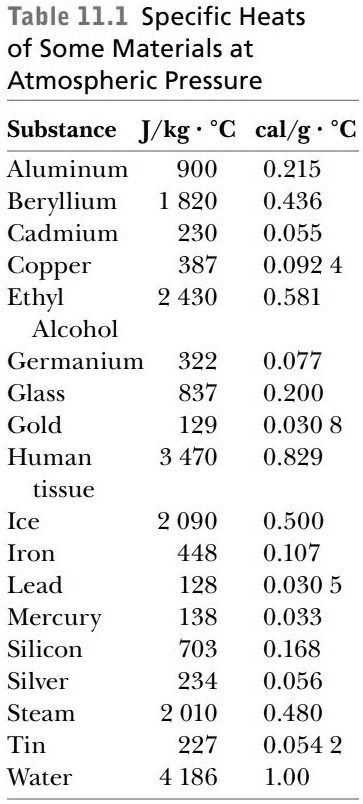
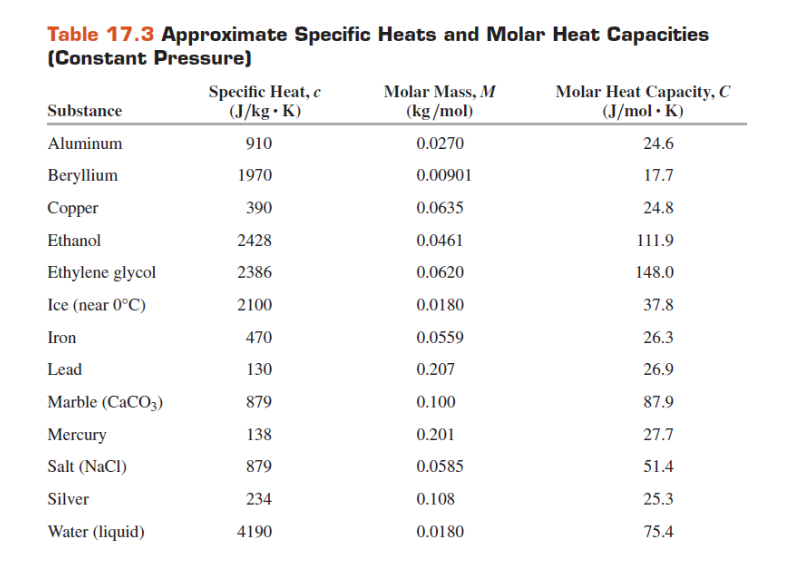
SOLVED: Table 11.1 Specific Heats of Some Materials at Atmospheric Pressure Substance J/kg 'C cal/g Aluminum 900 0.215 Beryllium 820 0.436 Cadmium 230 0.055 Copper 387 0.092 4 Ethyl 2 430 0.581

The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity
What is the difference between specific latent heat of melting of ice and specific latent heat of fusion of ice? - Quora

A 40 g ice cube (latent heat of fusion = 3.33 x 10^5 J/kg) floats in 200 g of water (specific heat = 4184 J/kg degree Celsius) in a 100 g copper (